Honour Chemistry Semester Break Reading
Assignment
 We
will be embarking on Atomic Orbitals, Chemical Periodicity, Bonding and
Structures when we return from the break. Hence, it is very important that you
read ahead to get an idea of what’s coming. Look over the math examples but
don’t fret them if you don’t understand. Try to comprehend the main ideas by
following the development of atomic and quantum science in the early part of the
20th century. Do this over several days (that means no cramming it
all in the night before we get back to school – this is not your typical summer
reading assignment for your English class). Enjoy the process of discovery and
learning as you try to digest these concepts.
We
will be embarking on Atomic Orbitals, Chemical Periodicity, Bonding and
Structures when we return from the break. Hence, it is very important that you
read ahead to get an idea of what’s coming. Look over the math examples but
don’t fret them if you don’t understand. Try to comprehend the main ideas by
following the development of atomic and quantum science in the early part of the
20th century. Do this over several days (that means no cramming it
all in the night before we get back to school – this is not your typical summer
reading assignment for your English class). Enjoy the process of discovery and
learning as you try to digest these concepts.
1. Go
through each point below and take in the main idea. Then, read the prescribed
section (s) from the textbook (ignore Diamagnetism, Paramagnetism and Shielding
Effect in Many-Electron Atoms on pg. 228 & 229). Go to the suggested websites as
you focus on the following ideas,.
a. Light
was thought of as waves for the longest time (the shorter the wavelength, the
higher the frequency because c = f λ), as it has wave properties
like reflection, refraction, and diffraction with interference pattern. The
higher the frequency or the shorter the wavelength, the stronger is the light
energy, E = hf or E = hc/λ.
Read 7.1 of the textbook (pg. 207 to 210).
b. Planck
hypothesized the quantum nature of light (discrete amount of energy) and
Einstein later proved by his Photoelectric Experiment.
Read 7.2 of the textbook (pg. 211 and 212).
A photoelectric device is basically an early version of a solar panel – play
with this
applet
by increasing and decreasing wavelength and intensity of light. This experiment
demonstrated that light can behave like a particle (photon) as well as a wave
(Einstein won the Noble Prize of Physics for this work). It led to the idea of
Wave-Particle Duality of Light.
c. Bohr
used Rutherford’s earlier work of the nuclear model,
applied the idea from Planck that light is quantized
(discrete in amounts of energy), and that electrons can only exist at specific
energy levels. Using mathematical proofs, he developed the Bohr Atomic
Model. The model explains how electrons can absorb quantized amounts of
energy. As these electrons relax back to their lower energy levels, they give
out lights of specific wavelengths – emission spectrum. Since different elements
have different number of electrons, their spectrums are as different as
fingerprints.
Read 7.3 of the textbook (pg. 212 to 217).
Look over the calculations in the examples (don’t worry; you won’t have to do
them on a test. But you must appreciate how the Bohr Model can mathematically
predict these energy levels of a hydrogen atom.) - See this
video and view this
animation. The Bohr Model is also called as the Shell
or Energy Level Model. It has a limitation in that it only work on hydrogen atoms, and
there are other problems the Bohr Model cannot explain. Most scientists now classified
the Bohr Model as the “old quantum theory”.
d. de
Broglie suggested
that matter can have wave properties (since wave can behave like particles as
Einstein had proved earlier). He developed an idea
an electron is not just a particle, but can also be viewed as waves (see the
first 3 ½ minutes of this video). Further evidence provided
by George Thomson (J. J Thomson’s son of the Plum Pudding
Model fame) using high energy electrons to “see” the diffraction pattern of a
crystal – precursor of the modern day electron microscope (see the
X-ray
Diffraction picture of a DNA molecule by Rosalind Franklin –
independent discoverer of DNA – she did not work with Crick and Watson). This
generalized into the Particle-Wave Duality of Electrons. The
equation [ λ = h/(mv) ] allows us to calculate the wavelength
of an electron moving at any velocity.
Read 7.4 of the textbook (pg. 217 to 219).
e. Bohr
understood that his shell
model was incomplete and fairly limited. Therefore, he decided to work with
Heisenberg who realized that electrons could not be observed
directly due to its extremely small size. More importantly, the “act” of
observing would inevitably change its position (because you would have to shine
a light with high frequency to “see” an electron, and it would unavoidably
absorb this energy and move to a higher energy level). Hence, there would always
be uncertainties associate with the position and momentum of an electron at any
given time. This concept is called the Heisenberg Uncertainty Principle.
Therefore, atomic orbitals must be thought of as probability clouds where
electrons are “likely” to be found. (See
PowerPoint slides for more explanation and watch this
excellent
demo.)
f. Since
electrons in different energy levels can now be thought of as wave according to
de Broglie, and not particles, Schrödinger
used a complex mathematical model to express these waves as a standing wave
functions (sort of like a sinusoidal wave you learned in Algebra 2, except this
is in 3-D) Watch
this video
of standing waves in a ring. (The audio is in Chinese but read the English
caption on the video.) Bohr and Heisenberg took
Schrödinger wave functions and applied them with their matrix based
probability model and developed the Probability Cloud (Density) Model.
Read 7.5 of the textbook (pg. 219 to 220).
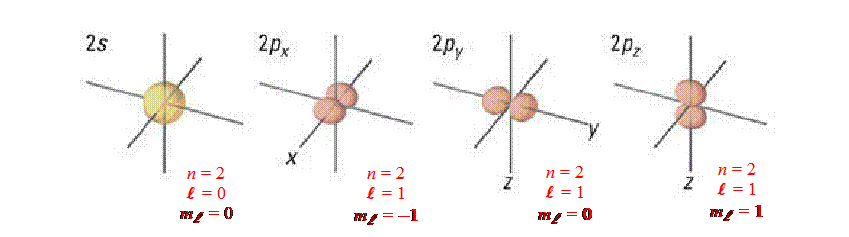
The solutions of these wave functions (or wave equations) are called the
Quantum Numbers. These quantum numbers allow us to generate different
cloud shapes and sizes (1s, 2s, 2p, 3s, 3p, 3d
… etc). They are called Atomic Orbitals.
-
n
– Principle Quantum Numbers
{n = 1, 2, 3, 4 …}. They represent the energy levels (or different sizes
of these orbitals)
-
l
– Angular Quantum Numbers
{l
= 0, 1, 2, 3, … , (n −
1)}. They represent the different types or shapes of the orbitals We use the
letters (s, p, d… etc.) to represent these numbers.
(l
= 0 is s-orbital – sphere); (l
= 1 is p-orbital – dumb bell); (l
= 2 is d-orbital –four-leaf clover)
Example:
For n = 2, there can be two types of orbitals (l
= 0 and
l
= 1) ®
so in energy level n = 2, there are two kinds of orbitals (2s and
2p).
The s-orbital has 1 orientation (0
≤ ml
≤ 0 ® ml
= 0 [one solution means only one orientation for s-orbital]).
The p-orbital has 2 orientations
(−1
≤ ml
≤ 1 ® ml
= −1, 0, and 1 [three solutions means only three orientations for p-orbital:
px, py and pz ]).
Now,
Read 7.6 and 7.7 of the textbook (pg. 221 to 226).
But if you can’t follow the book, then read the
notes on this website. Watch the
last 5 ½ minutes of this video.
g. Pauli
(a student / colleague of Heisenberg) understood the spin directions of the
electrons can give rise to different magnetic pole directions. Hence, there can
only be two electrons in any atomic orbital (see Fig. 7.22b on pg. 228 of
textbook).
Read the first half of 7.8 of the textbook (pg. 226 to 230).
Break Time: Watch the video clips from the
Documentary:
“Atom – The
Clash of Titans”
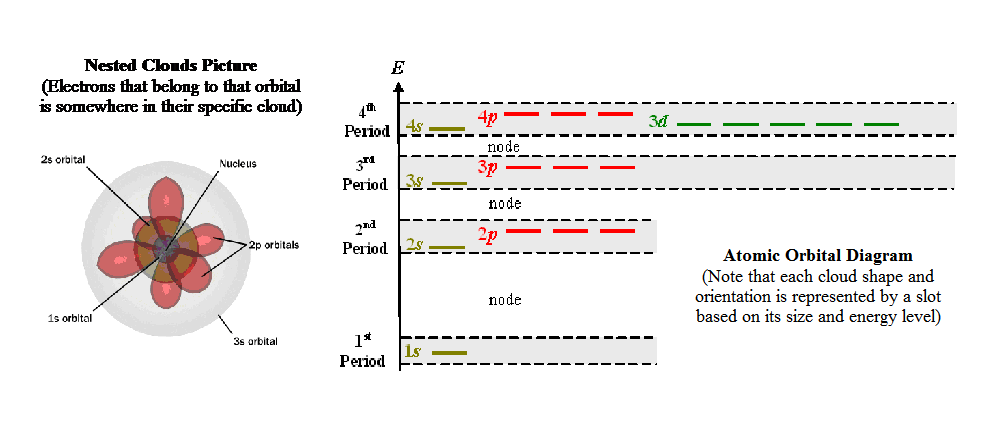
h. Even
though Heisenberg and Bohr would say it is impossible to
perceive an atom and their orbitals, it would still be nicer to have a
conceptual model (a picture if you will). Atomic orbitals can be drawn as
Nested Clouds (sort of like those Russian nested dolls - Babushka doll –
see diagram below), or they can be drawn as Atomic Orbital Diagrams
(slot boxes with arrows), or they can be written out as Electron
Configuration. (Please go through the online tutorial:
Electron Configurations - Orbital Filling Order.
(Use the Table of Elements on pg. 236 of the textbook at the same
time.) Also look over
this awesome
applet. It shows why the 3d orbitals degenerate or
move up above the 4s orbital due to electron crowding. This explains why
the elements in 3d block of the Periodic Table come after the 4s
elements, event though the 3d block is in the 4th row of the
table.
i. When
filling out the atomic orbitals, we have to follow the Aufbau Principle
and the Hund’s Rule.
Read the last half of 7.8 and 7.9 of the textbook (pg. 230 to 235).
Watch this
Lecture Video from MIT.
2. For
a recap and summarizing ideas as well as applications using quantum mechanics.
Watch
all three
video clips. Look over the printable flow chart
of the development of ideas that lead to the
Modern Quantum Mechanics Model. As you can see, it was a
collaborative result of many scientists. Remember, don’t get bog down with the
details, but understand the main ideas and developments of quantum mechanics
view of atoms.
Have a good Break, Mr. Tang
 We
will be embarking on Atomic Orbitals, Chemical Periodicity, Bonding and
Structures when we return from the break. Hence, it is very important that you
read ahead to get an idea of what’s coming. Look over the math examples but
don’t fret them if you don’t understand. Try to comprehend the main ideas by
following the development of atomic and quantum science in the early part of the
20th century. Do this over several days (that means no cramming it
all in the night before we get back to school – this is not your typical summer
reading assignment for your English class). Enjoy the process of discovery and
learning as you try to digest these concepts.
We
will be embarking on Atomic Orbitals, Chemical Periodicity, Bonding and
Structures when we return from the break. Hence, it is very important that you
read ahead to get an idea of what’s coming. Look over the math examples but
don’t fret them if you don’t understand. Try to comprehend the main ideas by
following the development of atomic and quantum science in the early part of the
20th century. Do this over several days (that means no cramming it
all in the night before we get back to school – this is not your typical summer
reading assignment for your English class). Enjoy the process of discovery and
learning as you try to digest these concepts.